Usuario:Rjgalindo/WikiProyecto/Doctrina

Alar Plate. The dorsal (posterior) half of the neural tube, separated by the basal plate by the sulcus limitans. It contains nuerons involved in the communication of general somatic and general visceral sensory impulses. The more caudal aspect becomes the sensory axon part of the spinal cord.
Allosteric enzymes. Their active site activity becomes modified by binding of a substance (ligand) at a different site. It could be another active site on the enzyme and the ligand can be any substrate, competitor or not. These enzymes tend to have more than one polypeptide and they usually are the enzymes that catalize the committed steps in a metabolyc pathway. Allosteric regulation doesn't involve altering covalent bonds.
Alpha-1 (α1) adrenergic receptor. Adrenergic receptor associated with the Gq-protein. It signals for catecholamines like norepinephrine and epinephrine (adrenaline). Four general action regions: constriction of muscles (cardiac, skeletal and smooth: including vasoconstriction and dilation of pupil-mydriasis-, opposed by β2-adrenergic receptors, which mediate vasodilation), contraction of uterus and bladder sphincter, and glycolisis and gluconeogenesis in the liver. Upon activation, α1-adrenergic receptors react with a heterotrimeric G protein, Gq, activates phospholipase C (PLC), which causes an increase in IP3 and calcium. This activates Protein Kinase C, which functions by phosphorylation of other enzymes causing their activation, or by phosphorylation of certain channels leading to the increase or decrease of electrolyte transfer in or out of the cell. Mnemonic: the α sign looks like a string ready to constrict by pulling on tips. Ligands:
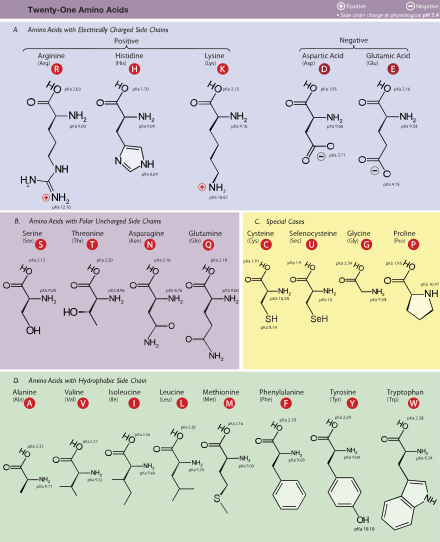
Amino acids.
- Amino acids with small non-polar side chains: Glycine, Alanine, Leucine, Valine, Isoleucine
- Amino acids with large non-polar side chains: Phenylalanine, Tyrosine and Tryptophan
- Amino acids with basic side chains: Histidine, Arginine and Lysine
- Amino acids with acid side chains: Aspartic acid and Glutamic acid
- Amino acids with non-ionic polar side chains: Glutamine, Asparagine, Serine, Threonine
- Amino acids with sulfur containing side chains: Methionine, Cysteine
- Proline: an imino acid
- Ornithine and S-adenosylmethionine are precursors of polyamines.[5]
| Essential | Nonessential |
|---|---|
| Histidine | Alanine |
| Isoleucine | Arginine* |
| Leucine | Asparagine |
| Lysine | Aspartic acid |
| Methionine | Cysteine* |
| Phenylalanine | Glutamic acid |
| Threonine | Glutamine* |
| Tryptophan | Glycine |
| Valine | Ornithine* |
| Proline* | |
| Selenocysteine* | |
| Serine* | |
| Taurine* | |
| Tyrosine* |
(*) Essential only in certain cases.[6][7]
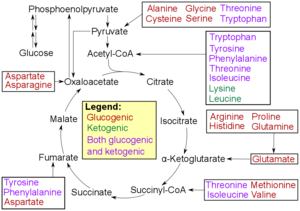
- Catabolism of aminoacids. Amino acids can be classified according to the properties of their main products as either of the following:[8]
- Glucogenic, with the products having the ability to form glucose by gluconeogenesis
- Ketogenic, with the products not having the ability to form glucose. These products may still be used for ketogenesis or lipid synthesis. Mnemonic: what comes after the K? All Ketogenic aminoacids begin with the letter L.
- Amino acids catabolized into both glucogenic and ketogenic products.
- Degradation of an amino acid often involves deamination by moving its amino group to alpha-ketoglutarate, forming glutamate. This process involves transaminases, often the same as those used in amination during synthesis. In many vertebrates, the amino group is then removed through the urea cycle and is excreted in the form of urea. However, amino acid degradation can produce uric acid or ammonia instead. For example, serine dehydratase converts serine to pyruvate and ammonia.[9] After removal of one or more amino groups, the remainder of the molecule can sometimes be used to synthesize new amino acids, or it can be used for energy by entering glycolysis or the citric acid cycle, as detailed in image at right.
| Amino Acid | Abbrev. | Remarks | |
|---|---|---|---|
| Alanine | A | Ala | Very abundant, very versatile. More stiff than glycine, but small enough to pose only small steric limits for the protein conformation. It behaves fairly neutrally, and can be located in both hydrophilic regions on the protein outside and the hydrophobic areas inside. |
| Asparagine or aspartic acid | B | Asx | A placeholder when either amino acid may occupy a position. |
| Cysteine | C | Cys | The sulfur atom bonds readily to heavy metal ions. Under oxidizing conditions, two cysteines can join together in a disulfide bond to form the amino acid cystine. When cystines are part of a protein, insulin for example, the tertiary structure is stabilized, which makes the protein more resistant to denaturation; therefore, disulfide bonds are common in proteins that have to function in harsh environments including digestive enzymes (e.g., pepsin and chymotrypsin) and structural proteins (e.g., keratin). Disulfides are also found in peptides too small to hold a stable shape on their own (e.g. insulin). |
| Aspartic acid | D | Asp | Behaves similarly to glutamic acid. Carries a hydrophilic acidic group with strong negative charge. Usually is located on the outer surface of the protein, making it water-soluble. Binds to positively-charged molecules and ions, often used in enzymes to fix the metal ion. When located inside of the protein, aspartate and glutamate are usually paired with arginine and lysine. Aspartate, glycine, and glutamine are precursors of nucleotides.[10] |
| Glutamic acid | E | Glu | Behaves similarly to aspartic acid. Has longer, slightly more flexible side chain. |
| Phenylalanine | F | Phe | Essential for humans. Phenylalanine, tyrosine, and tryptophan contain large rigid aromatic group on the side-chain. These are the biggest amino acids. Like isoleucine, leucine and valine, these are hydrophobic and tend to orient towards the interior of the folded protein molecule. Phenylalanine can be converted into Tyrosine. |
| Glycine | G | Gly | Because of the two hydrogen atoms at the α carbon, glycine is not optically active. It is the smallest amino acid, rotates easily, adds flexibility to the protein chain. It is able to fit into the tightest spaces, e.g., the triple helix of collagen. As too much flexibility is usually not desired, as a structural component it is less common than alanine. Glycine is a precursor of porphyrins such as heme.[11] |
| Histidine | H | His | In even slightly acidic conditions protonation of the nitrogen occurs, changing the properties of histidine and the polypeptide as a whole. It is used by many proteins as a regulatory mechanism, changing the conformation and behavior of the polypeptide in acidic regions such as the late endosome or lysosome, enforcing conformation change in enzymes. However only a few histidines are needed for this, so it is comparatively scarce. |
| Isoleucine | I | Ile | Essential for humans. Isoleucine, leucine and valine have large aliphatic hydrophobic side chains. Their molecules are rigid, and their mutual hydrophobic interactions are important for the correct folding of proteins, as these chains tend to be located inside of the protein molecule. |
| Leucine or isoleucine | J | Xle | A placeholder when either amino acid may occupy a position |
| Lysine | K | Lys | Essential for humans. Behaves similarly to arginine. Contains a long flexible side-chain with a positively-charged end. The flexibility of the chain makes lysine and arginine suitable for binding to molecules with many negative charges on their surfaces. E.g., DNA-binding proteins have their active regions rich with arginine and lysine. The strong charge makes these two amino acids prone to be located on the outer hydrophilic surfaces of the proteins; when they are found inside, they are usually paired with a corresponding negatively-charged amino acid, e.g., aspartate or glutamate. |
| Leucine | L | Leu | Essential for humans. Behaves similar to isoleucine and valine. See isoleucine. |
| Methionine | M | Met | Essential for humans. Always the first amino acid to be incorporated into a protein; sometimes removed after translation. Like cysteine, contains sulfur, but with a methyl group instead of hydrogen. This methyl group can be activated, and is used in many reactions where a new carbon atom is being added to another molecule. |
| Asparagine | N | Asn | Similar to aspartic acid. Asn contains an amide group where Asp has a carboxyl. |
| Pyrrolysine | O | Pyl | Similar to lysine, with a pyrroline ring attached. |
| Proline | P | Pro | Contains an unusual ring to the N-end amine group, which forces the CO-NH amide sequence into a fixed conformation. Can disrupt protein folding structures like α helix or β sheet, forcing the desired kink in the protein chain. Common in collagen, where it often undergoes a posttranslational modification to hydroxyproline. |
| Glutamine | Q | Gln | Similar to glutamic acid. Gln contains an amide group where Glu has a carboxyl. Used in proteins and as a storage for ammonia. The most abundant Amino Acid in the body. |
| Arginine | R | Arg | Functionally similar to lysine. Arginine is a precursor of nitric oxide.[12] |
| Serine | S | Ser | Serine and threonine have a short group ended with a hydroxyl group. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in enzymes. Both are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them. |
| Threonine | T | Thr | Essential for humans. Behaves similarly to serine. |
| Selenocysteine | U | Sec | Selenated form of cysteine, which replaces sulfur. |
| Valine | V | Val | Essential for humans. Behaves similarly to isoleucine and leucine. See isoleucine. |
| Tryptophan | W | Trp | Essential for humans. Behaves similarly to phenylalanine and tyrosine (see phenylalanine). Naturally fluorescent. Tryptophan is a precursor of the neurotransmitter serotonin.[13] |
| Unknown | X | Xaa | Placeholder when the amino acid is unknown or unimportant. |
| Tyrosine | Y | Tyr | Behaves similarly to phenylalanine (precursor to Tyrosine) and tryptophan (see phenylalanine). Precursor of melanin, epinephrine, the neurotransmitter dopamine and thyroid hormones. Naturally fluorescent, although fluorescence is usually quenched by energy transfer to tryptophans. |
| Glutamic acid or glutamine | Z | Glx | A placeholder when either amino acid may occupy a position. |
Arachnoid. It's the middle of the meninges and non vascular.
Arginine. An essential aminoacid for children, especially preterm infants,[14] is a precursor of NO. Arginine is synthesized from citrulline by argininosuccinate synthetase (ASS) and argininosuccinate lyase, a costly reaction, as it requires hydrolysis of two ATP to AMP. Synthesis of arginine occurs principally in the small intestine, which produce citrulline primarily from glutamine and glutamate, and then the proximal tubule cells of the kidney extract citrulline from the circulation and convert it to arginine, which is returned to the circulation. As a consequence, impairment of small bowel or renal function can reduce endogenous arginine synthesis, thereby increasing the dietary requirement.
Aromatic aminoacids. All hydrophilic aminoacids: Phenylalaine, Histidine, Tryptophan (all three essencial aminoacids) and Tyrosine (semi-essential since it is synthesized from an essential aminoacid). The only difference between Phenylalanine and Tyrosine is an OH group, so enzyme hydroxylase is all that is needed to make Tyrosine from Phenylalanine, the enzyme is deficient in PKU. Tyrosine is a precursor of melanin and catecholamines. Tryptophan is a precursor of serotonin and niacin (deficient in Hartnup disease). Triptophan also makes melatonin important for the circadian rithm.

Basal Plate. Part of the neural tube, extending from the mesencephalon (midbrain) down to the end of the spinal chord. It is located in the ventral half of the neural tube separated from the Alar Plate by the sulcus limitans. Contains primarily motor neurons.
Beta-2 (β2) adrenergic receptor. Cellular receptor directly associated with one of its ultimate effectors, the class C L-type calcium channel, coupled to the Gs G protein, which activates adenylyl cyclase, catalysing the formation of cyclic adenosine monophosphate (cAMP) which then activates protein kinase A.[15] Beta-2 Adrenergic Receptors have also been found to couple with Gi, possibly providing a mechanism by which response to ligand is highly localized within cells because in contrast, Beta-1 Adrenergic Receptors are coupled only to Gs, which results in a more diffuse cellular response.[16] This appears to be mediated by cAMP induced PKA phosphorylation of the receptor.[17] Actions of the β2 receptor include:
| Tissue/Effect | Muscle effect | |
|---|---|---|
|
Smooth muscle relaxation in: |
||
| GI tract (decreases motility) | Delay digestion during fight-or-flight response | |
|
detrusor urinae muscle of bladder wall[18] This effect is stronger than the alpha-1 receptor effect of contraction. |
Delay need of micturition | |
| seminal tract[19] | ||
| bronchi[20] | Facilitate respiration (agonists can be useful in treating asthma) | |
|
Increase perfusion of organs | needed during fight-or-flight |
| striated muscle | Tremor[19] (via PKA mediated facilitation of presynaptic Ca2+ influx leading to acetylcholine release) | |
| Increased mass and contraction speed[19] | fight-or-flight | |
| glycogenolysis[19] | provide glucose fuel | |
- Circulatory system
- Increase cardiac output (minor degree compared to β1).
- Increase heart rate [20] in sinoatrial node (SA node) (chronotropic effect).
- Increase atrial cardiac muscle contractility. (inotropic effect).
- Increases contractility and automaticity[20] of ventricular cardiac muscle.
- Dilate hepatic artery.
- Dilate arteries to skeletal muscle.
- Increase cardiac output (minor degree compared to β1).
- Eye: in glaucoma, drainage is reduced ( open-angle glaucoma) or blocked completely (closed-angle glaucoma). In such cases, beta-2 stimulation with its consequent increase in humour production is highly contra-indicated, and conversely, a topical beta-2 antagonist such as timolol may be employed. In the normal eye, beta-2 stimulation by salbutamol increases intraocular pressure via net:
- Increase in production of aqueous humour by the ciliary process,
- Subsequent increased pressure-dependent uveoscleral outflow of humour, despite reduced drainage of humour via the Canal of Schlemm.
- Digestive system
- Glycogenolysis and gluconeogenesis in liver.[20]
- Glycogenolysis and lactate release in skeletal muscle.[20]
- Contract sphincters of GI tract.
- Insulin secretion from pancreas.[20][22]
- Thickened secretions from salivary glands.[20]
- Other:
- Inhibit histamine-release from mast cells.
- Increase protein content of secretions from lacrimal glands.
- Increase renin secretion from kidney.
- Receptor also present in cerebellum.
- Bronchiole dilation (targeted while treating asthma attacks)
- Involved in brain - immune - communication [23]
- Agonists: Muscle relaxants in asthma and COPD
- salbutamol (albuterol in USA)
- bitolterol mesylate
- isoproterenol
- levosalbutamol (levalbuteral in USA)
- metaproterenol
- formoterol
- salmeterol
- terbutaline
- clenbuterol
- ritodrine (tocolytic)
- Antagonists

Brain. Along with the Spinal Cord, forms the Central Nervous System. The brain is formed from the Neural Tube: Motor Neurons are formed from the Basal Plate; Sensory Neurons are formed by the Alar Plate; the Basal and Alar Plates are separated from each other by the Sulcus Limitans. Pathways within the brain: Trigeminothalamic pathway, Corticobulbar tract and Vestibulocochlear Pathways (see also, Cerebellar Pathway and Vestibular Pathway as well as important pathways of the spinal cord). Associated with malformations: Anencephaly; conditions: Arnold-Chiari Syndrome, Hydrocephalus, Fetal Alcohol Syndrome.
Blood Brain Barrier. Continuous membrane composed by the arachnoid, the modified ependimal cells (ependyma is the epithelial cells that line the ventricular system) of the choroid plexus (which produces CSF) and the capillary endothelia.
Branched-chain amino acids. Refers to the amino acids having non-linear side-chains; these are leucine, isoleucine, and valine. Proline is the only amino acid whose side-group links to the α-amino group and.[25]
Citrulline. Citrulline can be derived from arginine via nitric oxide synthase (NOS), from ornithine via catabolism of proline or glutamine/glutamate or from asymmetric dimethylarginine (ADMA) via DDAH. Citrulline is made in the small intestine and recirculates by being converted to arginine in the kidney. It is a key intermediate in the urea cycle. Although it is considered an amino acid, citrulline is not coded for by DNA directly. Several proteins, including myelin basic protein (MBP), filaggrin, and several histone proteins, are known to contain citrulline as a result of a posttranslational modification. Other proteins, such as fibrin and vimentin are susceptible to citrullination during cell death and tissue inflammation. Citrullinemia is an autosomal recessive disorder that causes increase ammonia in the body.
Competitive inhibition. Competes with the normal substrate for the active binding site of the enzyme.
Dura mater. The outtermost of the meninges, tough connective tissue.
Dynein. A motor proteins that moves along microtubule filaments, powered by the hydrolysis of ATP and responsible for rapid axonal transport. They move towards the minus end of the microtubule. Thus they transport cargo from the periphery of the cell towards the centre, for example from the terminal buttons of a neuronal axon to the cell body (soma). This is known as retrograde transport.[26] Dynein causes sliding of microtubules in the axonemes of cilia and flagella. Cytoplasmic dynein performs functions such as organelle transport and centrosome assembly.[27] moving processively along the microtubule; that is, one or the other of its stalks is always attached to the microtubule so that the dynein can "walk" a considerable distance along a microtubule without detaching. Dynein probably helps to position the Golgi complex and other organelles in the cell.[27] It also helps transport cargo needed for cell function such as vesicles made by the endoplasmic reticulum, endosomes, and lysosomes. Dynein is involved in the movement of chromosomes and positioning the mitotic spindles for cell division.[28] Dynein carries organelles and microtubule fragments along the axons of neurons in a process called axoplasmic transport.[27]
Epidural Space. It's the outtermost space between the skull bone and the dura mater. It contains the middle meningeal artery and other arteries. An epidural hematoma will happen when these arteries tear with skull fractures.
Forebrain. The most cephalic derivative of the embryonic structures in the nervous system. Divided into two aspects, the Telencephalon and the Diencephalon.
Glycine. A hydrophobic aminoacid and an inhibitory neurotransmitter, particularly in the spinal cord. The smallest aminoacid, only a hydrogen as a side chain.
Gut tube. An endoderm-derived structure and can be divided into three segments: foregut, midgut, and hindgut. Components derived from the gut proper, including the stomach and colon, develop as swellings or dilatations of the primitive gut. In contrast, the structures that derive from the primitive gut but are not part of the gut proper, in general develop as out-pouchings of the primitive gut. The blood vessels supplying these structures remain constant throughout development.[29]
| Part | Part in adult | Gives rise to | Arterial supply |
|---|---|---|---|
| Foregut | Esophagus to first 2 sections of the duodenum | Esophagus, Stomach, Duodenum (1st and 2nd parts), Liver, Gallbladder, Pancreas, Superior portion of pancreas (Note that though the Spleen is supplied by the celiac trunk, it is derived from dorsal mesentery and therefore not a foregut derivative) |
celiac trunk |
| Midgut | lower duodenum, to the first two-thirds of the transverse colon | lower duodenum, jejunum, ileum, cecum, appendix, ascending colon, and first two-thirds of the transverse colon | branches of the superior mesenteric artery |
| Hindgut | last third of the transverse colon, to the upper part of the anal canal | last third of the transverse colon, descending colon, rectum, and upper part of the anal canal | branches of the inferior mesenteric artery |
Hemoglobin. Oxygen carrier, rich in alpha helix and contains heme whose iron atom is the site for oxygen binding: just like myoglobin. Hemoglobin is unique in that it is found in red cells, has four polypeptide chains, oxygen binding produces a sigmoidal curved graph, it can also carry CO2 and bind 2,3 diphosphoglycerate (which lowers oxygen affinity) and has positive cooperativity between the four chains. Fetal hemoglobin (2 alpha and 2 gamma) binds oxygen better than does the adult hemoglobin. Altered beta chain produces sickle cell anemia.
Hindbrain. The most caudal derivative of the embryonic structures in the nervous system. Divided into two aspects, the Metencephalon and the Myelencephalon. Think of alphabetically, the metencephalon is before the myelencephalon. Don't mix up with the mesencephalon (midbrain) which is higher up than the hindbrain (alphabetically, mesencephalon is first of all three m derivatives).
Myoglobin. Oxygen carrier, rich in alpha helix and contains heme whose iron atom is the site for oxygen binding: just like hemoglobin. Myoglobin is unique in that it is found in muscle cells, has only one polypeptide chain, oxygen binding produces a hyperbolic graph, has a higher affinity for O2 than hemoglobin and has no allosteric binding properties.
Phenylalaine. Along with Histidine, Tryptophan (all three essencial amino acids) and Tyrosine are aromatic amino acids. The only difference between Phenylalanine and Tyrosine is an OH group, so enzyme hydroxylase is all that is needed to make Tyrosine from Phenylalanine, the enzyme is deficient in PKU.
Histidine. An essential aminoacid, precursor of histamine and has a role in the treatment of peptic ulcer disease because of its effect on acid secretion.
Histones. Proteins rich in Lysine and Arginine because of the positive aminoacids allowing tight folding around DNA.
Hydrophilic aminoacids. Have charges in the side chain. Positive charge comes from an extra nitrogen. Negative charged because of an extra carboxil group making excitatory neurotransmiters. Felbamate and ketamine block glutamate receptors. Non charged hydrophilic aminoacids: Serine and threonine have an OH group that confers the property of O=glycosilation and O-phosphorilation. Cystein and Methionine have slufur in their side chain. The SH group of cysteine make disulfide bonds. Cysteine is part of gluthathione, used to detoxify free radicals, especially for neurodegenerative disorders (N-acetylcysteine), cystic fibrosis to dirupt mucus, hemorrhagic cystitis of cyclophosphamide and acetaminophen toxicity. Methionine has a methyl group and Sulfur, is a methyl donor as s-adenosyl methionine, for mRNA capping, making epineprhine from norepinephrine. Asparagine and glutamine have an extra amino group with no charges, so are good for N-glycosylation in the endoplasmic reticulum requiring dolicophosphate and intermediate in cholesterol.

Kinesin. A motor proteins that moves along microtubule filaments, powered by the hydrolysis of ATP (thus kinesins are ATPases). This peculiar movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo from the centre of the cell towards the periphery. This form of transport is known as anterograde transport.[26] Motor proteins fulfill the role of transporting large cargo about the cell to their required destinations. Kinesins are motor proteins that transport such cargo by walking unidirectionally along microtubule tracks hydrolysing one molecule of adenosine triphosphate (ATP) at each step.[30] It was thought that ATP hydrolysis powered each step, the energy released propelling the head forwards to the next binding site.[31] However, it has been proposed that the head diffuses forward and the force of binding to the microtubule is what pulls the cargo along.[32] Dyneins, move towards the minus end of the microtubule.
Leucine. A strictly ketogenic aminoacid, meaning it
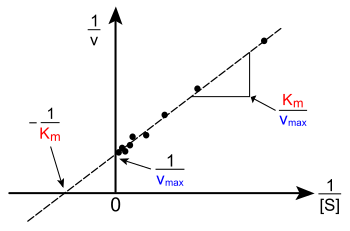
Lineweaver–Burk plot (or double reciprocal plot). A graphical representation of the Lineweaver–Burk equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934.[33] The plot provides a useful graphical method for analysis of the Michaelis–Menten equation:
Taking the reciprocal gives
where V is the reaction rate (velocity), Km is the Michaelis–Menten constant, Vmax is the maximum reaction velocity, and [S] is the substrate concentration.
The Lineweaver–Burk plot was widely used to determine important terms in enzyme kinetics, such as Km and Vmax, before the wide availability of powerful computers and non-linear regression software. The y-intercept of such a graph is equivalent to the inverse of Vmax; the x-intercept of the graph represents −1/Km. It also gives a quick, visual impression of the different forms of enzyme inhibition. Lineweaver–Burk plot distorts the error features of the data, and it is therefore unreliable for the determination of enzyme kinetic parameters. Although it is still used for representation of kinetic data,[34] non-linear regression or alternative linear forms of the Michaelis–Menten equation.[35] When used for determining the type of enzyme inhibition, the Lineweaver–Burk plot can distinguish competitive, non-competitive and uncompetitive inhibitors. Competitive inhibitors have the same y-intercept as uninhibited enzyme (since Vmax is unaffected by competitive inhibitors the inverse of Vmax also doesn't change) but there are different slopes and x-intercepts between the two data sets. Non-competitive inhibition produces plots with the same x-intercept as uninhibited enzyme (Km is unaffected) but different slopes and y-intercepts. Uncompetitive inhibition causes different intercepts on both the y- and x-axes but the same slope.
Proline. An aminoacid that disrupts alpha helices due to its cyclic side chain, allowing the protein to change shape.
Mesencephalon. Another name for midbrain. Do not confuse with Metencephalon, which is lower in the hindbrain area. Mnemonic, alphabetically, mesencephalon is before metenchephalon.
Metencephalon. The higher (cephalic) half of the hindbrain, between the mesencephalon and the myelencephalon. Will form two structures (the only derivative to form two structures): the cerebellum and the pons.
Midbrain. The middle derivative of the embryonic structures in the nervous system. Also called mesencephalon and will give up the midbrain.
Myelencephalon. The lower (most caudal) aspect of the hindbrain, will become the medulla. Mnemonic: See the y and the u, how close they are to each other alphabetically? As in myelencephalon and medulla.
Nissl substance. Fancy name for the Rough Endoplasmic Reticulum in neurons. Tends to stay in the axonal body without extending into the axon.
Pia matter. Thin, innermost of the meninges, covers the brain and the spinal cord, and it is highly vascular.
Subarachnoid Space. It's the space between the arachnoid and pia mater and contains the CSF. A subarachnoid hematoma can ocurr when there's rupture of a berry aneurysm. This is the space that CSF is drained for a lumbar puncture, between L3-L4 or L4-L5 disks.
Subdural Space. It's the space between the dura and aracnoid meningeal membranes. A subdural hematoma can happen when veis rupture within the subdural space.
Telencephalon. The most cephalic aspect of the Forebrain (procencephalon).
Tyrosine. A precursor of melanin (pigmentation of tissues), cathecolamines (dopamine, norepinephrine and epinephrine) because of tyrosine precursor places that have catecholamines are colored such as sustancia nigra and the norepinephrine area in the brainstem locus cereus (blue). Tyrosinase (requires copper) is the enzyme deficient in albinism. Tyrosine also in thyroglobulin is iodinated to form iodothyrosine T3 and T4.
Tryptophan. A precursor of serotonin and niacin. Hartnup disease has low tryptophan and low niacin. Tryptophan also makes melatonin important for the circadian rithm. N terminal signal sequence encodes hydrophobic aminoacids. N terminal signal sequence encodes hydrophobic aminoacids.
Zymogen. An inactive enzyme precursor, once
References[editar]
- ↑ Erman MK, Rosenberg R, For The U S Modafinil Shift Work Sleep Disorder Study Group. "Modafinil for excessive sleepiness associated with chronic shift work sleep disorder: effects on patient functioning and health-related quality of life." Primary Care Companion to the Journal of Clinical Psychiatry 2007;9(3):188-94. Full Text
- ↑ Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, Arora S, Schwartz JRL, Niebler GE, Dinges DF (August de 2005). «Modafinil for Excessive Sleepiness Associated with Shift-Work Sleep Disorder». N Engl J Med 353 (5): 476-486. PMID 16079371. doi:10.1056/NEJMoa041292.
- ↑ "Provigil (Modafinil) Site." 24 December 1998 [1].
- ↑ Fahed S, Grum DF, Papadimos TJ (2008). «Labetalol infusion for refractory hypertension causing severe hypotension and bradycardia: an issue of patient safety». Patient Saf Surg 2: 13. PMC 2429901. PMID 18505576. doi:10.1186/1754-9493-2-13.
- ↑ Rodríguez-Caso C, Montañez R, Cascante M, Sánchez-Jiménez F, Medina MA (August de 2006). «Mathematical modeling of polyamine metabolism in mammals». The Journal of Biological Chemistry 281 (31): 21799-812. PMID 16709566. doi:10.1074/jbc.M602756200.
- ↑ Fürst P, Stehle P (June de 2004). «What are the essential elements needed for the determination of amino acid requirements in humans?». The Journal of Nutrition 134 (6 Suppl): 1558S-1565S. PMID 15173430.
- ↑ Reeds PJ (July de 2000). «Dispensable and indispensable amino acids for humans». The Journal of Nutrition 130 (7): 1835S-40S. PMID 10867060.
- ↑ Stipanuk, M. H. (2006). Biochemical, physiological, & molecular aspects of human nutrition (2 ed.): Saunders Elsevier.
- ↑ Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2002). Biochemistry. San Francisco: W.H. Freeman. pp. 639-49. ISBN 0-7167-4684-0.
- ↑ Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2002). Biochemistry. San Francisco: W.H. Freeman. pp. 693-8. ISBN 0-7167-4684-0.
- ↑ Shemin D, Rittenberg D (1 December 1946). «The biological utilization of glycine for the synthesis of the protoporphyrin of hemoglobin». Journal of Biological Chemistry 166 (2): 621
|página=y|páginas=redundantes (ayuda). PMID 20276176. - ↑ Tejero J, Biswas A, Wang ZQ, et al. (November de 2008). «Stabilization and characterization of a heme-oxy reaction intermediate in inducible nitric-oxide synthase». The Journal of Biological Chemistry 283 (48): 33498-507. PMC 2586280. PMID 18815130. doi:10.1074/jbc.M806122200.
- ↑ Savelieva KV, Zhao S, Pogorelov VM, et al. (2008). «Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants». En Bartolomucci, Alessandro, ed. PloS ONE 3 (10): e3301. Bibcode:2008PLoSO...3.3301S. PMC 2565062. PMID 18923670. doi:10.1371/journal.pone.0003301.
- ↑ Wu, G.; et al. (August de 2004). «Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications». Journal of Nutritional Biochemistry 15 (8): 332-451 REVIEW. PMID 15302078. doi:10.1016/j.jnutbio.2003.11.010.
- ↑ Rubenstein LA, Zauhar RJ, Lanzara RG (2006). «Molecular dynamics of a biophysical model for β2-adrenergic and G protein-coupled receptor activation». J. Mol. Graph. Model. 25 (4): 396-409. PMID 16574446. doi:10.1016/j.jmgm.2006.02.008.
- ↑ Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG (November de 2000). «G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels». Biophys. J. 79 (5): 2547-56. Bibcode:2000BpJ....79.2547C. PMC 1301137. PMID 11053129. doi:10.1016/S0006-3495(00)76495-2.
- ↑ Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ (August de 2002). «Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system». J. Biol. Chem. 277 (34): 31249-56. PMID 12063255. doi:10.1074/jbc.M202753200.
- ↑ von Heyden B, Riemer RK, Nunes L, Brock GB, Lue TF, Tanagho EA (1995). «Response of guinea pig smooth and striated urethral sphincter to cromakalim, prazosin, nifedipine, nitroprusside, and electrical stimulation». Neurourol. Urodyn. 14 (2): 153-68. PMID 7540086. doi:10.1002/nau.1930140208.
- ↑ a b c d e Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 163
- ↑ a b c d e f g Fitzpatrick, David; Purves, Dale; Augustine, George (2004). «Table 20:2». Neuroscience (Third edición). Sunderland, Mass: Sinauer. ISBN 0-87893-725-0.
- ↑ Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 270
- ↑ Trovik TS, Vaartun A, Jorde R, Sager G (1995). «Dysfunction in the beta 2-adrenergic signal pathway in patients with insulin dependent diabetes mellitus (IDDM) and unawareness of hypoglycaemia». Eur. J. Clin. Pharmacol. 48 (5): 327-32. PMID 8641318. doi:10.1007/BF00194946.
- ↑ Elenkov, I. J., R. L. Wilder, et al. (2000). «The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system.». Pharmacol Rev 52 (4): 595-638. PMID 11121511.
- ↑ Goodlett, C.R., and Horn, K.H. Mechanisms of alcohol–induced damage to the developing nervous system. Alcohol Research & Health 25(3):175–184, 2001.
- ↑ Creighton, Thomas H. (1993). «Chapter 1». Proteins: structures and molecular properties. San Francisco: W. H. Freeman. ISBN 978-0-7167-7030-5.
- ↑ a b Vale RD (February de 2003). «The molecular motor toolbox for intracellular transport». Cell 112 (4): 467-80. PMID 12600311. doi:10.1016/S0092-8674(03)00111-9.
- ↑ a b c Gerald Karp, Kurt Beginnen, Sebastian Vogel, Susanne Kuhlmann-Krieg (2005). Molekulare Zellbiologie (en francés). Springer. ISBN 978-3-540-23857-7.
- ↑ Kiyomitsu, Tomomi; Iain M. Cheeseman (12 de febrero de 2012). «Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation». Nature Cell Biology. ISSN 1476-4679 1465-7392, 1476-4679
|issn=incorrecto (ayuda). doi:10.1038/ncb2440. Consultado el 14 de febrero de 2012. - ↑ Bruce M. Carlson (2004). Human Embryology and Developmental Biology (3rd edición). Saint Louis: Mosby. ISBN 0-323-03649-X.
- ↑ Schnitzer MJ, Block SM (1997). «Kinesin hydrolyses one ATP per 8-nm step». Nature 388 (6640): 386-390. Bibcode:1997Natur.388..386S. PMID 9237757. doi:10.1038/41111.
- ↑ Vale RD, Milligan RA (April de 2000). «The way things move: looking under the hood of molecular motor proteins». Science 288 (5463): 88-95. Bibcode:2000Sci...288...88V. PMID 10753125. doi:10.1126/science.288.5463.88.
- ↑ Mather WH, Fox RF (October de 2006). «Kinesin's biased stepping mechanism: amplification of neck linker zippering». Biophys. J. 91 (7): 2416-26. Bibcode:2006BpJ....91.2416M. PMC 1562392. PMID 16844749. doi:10.1529/biophysj.106.087049.
- ↑ Lineweaver, H and Burk, D. (1934). «The Determination of Enzyme Dissociation Constants». Journal of the American Chemical Society 56 (3): 658-666. doi:10.1021/ja01318a036.
- ↑ Hayakawa, K.; Guo, L.; Terentyeva, E.A.; Li, X.K.; Kimura, H.; Hirano, M.; Yoshikawa, K.; Nagamine, T. et al. (2006). «Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and Lactobacillus casei (Shirota)». J Chromatogr B Analyt Technol Biomed Life Sci 844 (2): 240-50. PMID 16876490. doi:10.1016/j.jchromb.2006.07.006.
- ↑ Greco, W. R. and Hakala, M. T., (1979). «Evaluation of methods for estimating the dissociation constant of tight binding enzyme inhibitors,» (PDF). J. Biol. Chem. 254 (23): 12104-12109. PMID 500698.

![{\displaystyle V=V_{\max }{\frac {[S]}{K_{m}+[S]}}.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/deede9c3fd432c251b72cd7b280ad3378ca33284)
![{\displaystyle {1 \over V}={{K_{m}+[S]} \over V_{\max }[S]}={K_{m} \over V_{\max }}{1 \over [S]}+{1 \over V_{\max }}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/262e1440a8ad30a692b153178eabbf6e7f45d48f)